Sona GNRs in targeted hyperthermia may create the opportunity to treat cancer without doing significant harm to healthy cells
Sona has engineered the right ‘Gene pool’ to pursue its Cancer Therapy
Over the previous 18 months the board has been changed to reflect the focus of the company on developing nano medicine for cancer therapy.

Interested in Investing?
- Join Sona Nanotech's Investor List
Why Sona Nanotech should be on your radar
Sona’s Biocompatible Nanoparticle Technology is Being Used to Develop Cancer Therapies & Rapid Lateral Flow Diagnostics. They play a key role in the advancement of impactful scientific and medical applications.
- Sona is developing Targeted Hyperthermia Therapy (THT) to shrink tumors without Surgery, Chemo or Radiation.
- THT uses Sona' biocompatible gold nanorods (GNRs) with an Infrared light source to deliver targeted heat to cancer cells, destroying cancer cells while healthy cells can go undamaged.
- It is expected that Sona’s gold nanotechnologies may be adapted for use in applications, as a safe and effective delivery system for multiple medical treatments, subject to the approval of various regulatory boards, including Health Canada and the FDA.

Why Sona should be on your radar
Sona’s Biocompatible Nanoparticle Technology is Being Used to Develop Cancer Therapies & Rapid Diagnostics. Sona’s unique gold nanorods have the potential to power the next generation of lateral flow diagnostics and play a key role in the advancement of impactful scientific and medical applications. Son’s GNRs are:
- CTAB-Free gold nanorods making it easier to use in vivo
- Can convert infrared light energy into heat in the same way as CTAB-based GNRs.
- Nanorods by Sona comes in a variety of lengths and widths to increase surface area and long shelf life and stable surface properties


Independent assessments from the Nanotechnology Characterization Laboratory (NCL)
- 1st: Determined that endotoxins and microbial contamination were “undetectable” based on both turbidity and chromogenic limulus amebocyte lysate (“LAL”) assays and the NCL’s endotoxin limit
- 2nd: Sona’s gold nanorods for consistency of physiochemical characterization and microbial contamination and endotoxin levels.
- 3rd: Showed that the continued improvements in Sona’s manufacturing process for GNRs have resulted in a significant reduction in free surfactant levels in nanorod dispersions, thereby minimizing the residual surfactant, yielding material better suited for use in vivo.
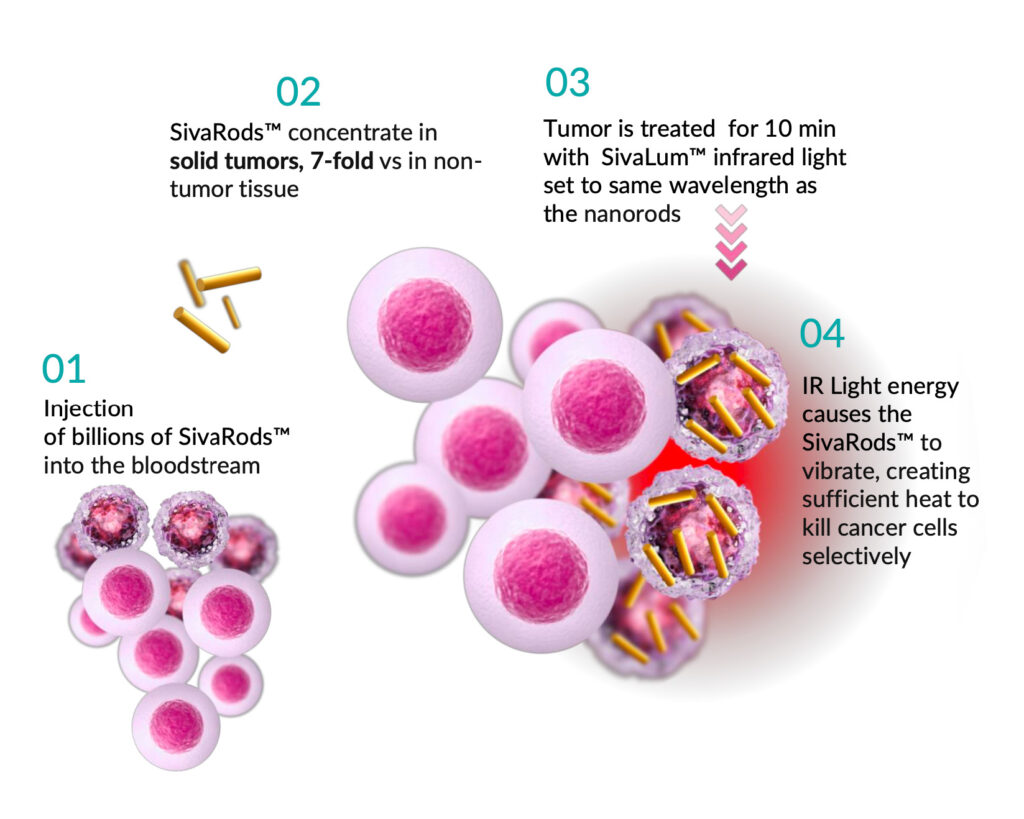
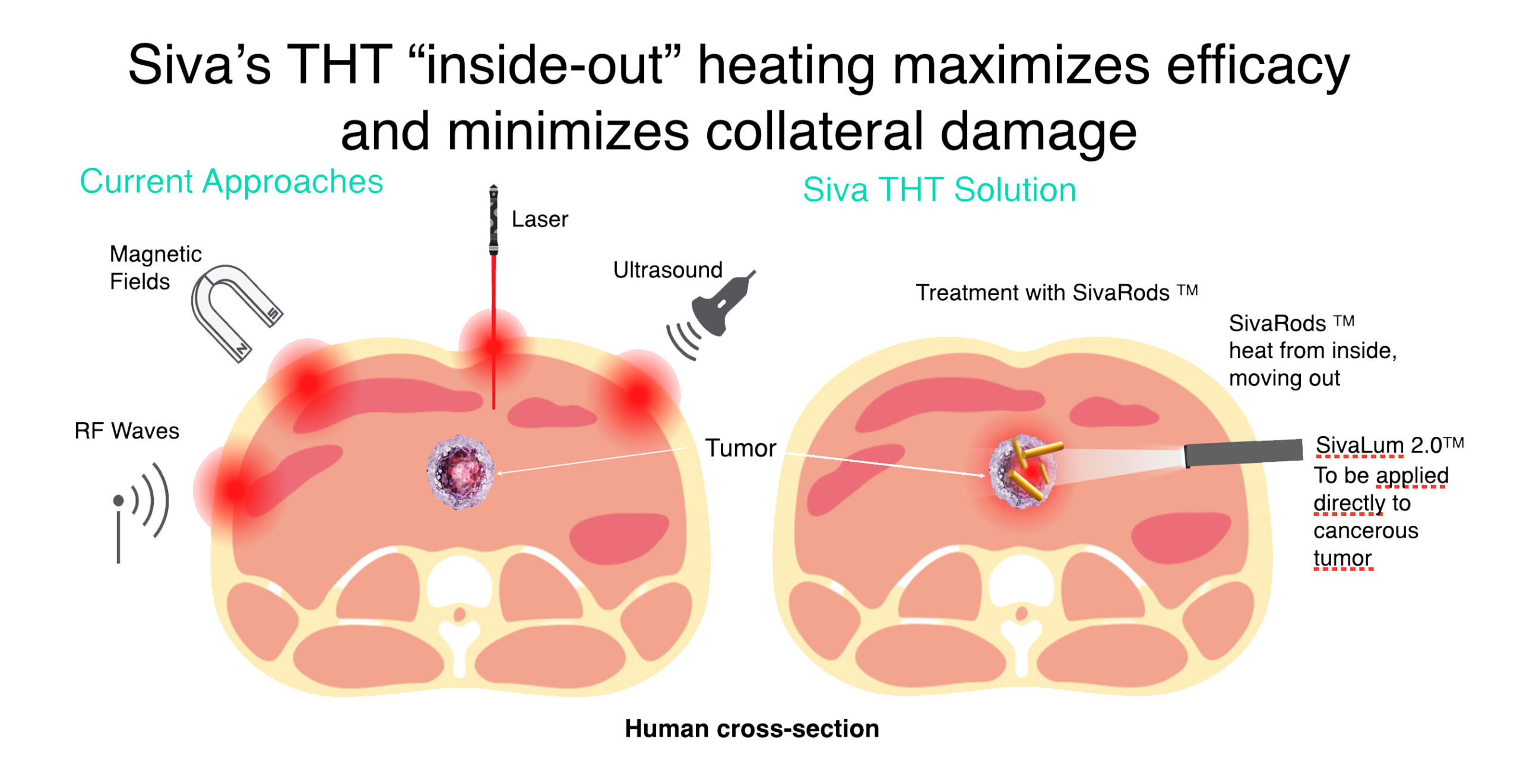
How does Targeted Hyperthermia Therapy (THT) work?
- Targets heat directly to the tumor
- Uses IV injection of GNRs to heat the tumor from the inside
- Achieves hyperthermia instead of ablation destroying cancerous cells selectively
- Sona GNRs are inert and do not use toxic CTAB
Healthy cells withstand heat stress, up to 52°C, typical with ablation therapies. Not damaged by hyperthermia’s 44°C.
SivaRods™ heat to 44°C and selectively kills cancer cells. It works from the inside of the tumor out . Heat shock protein (HSP) synthesis used
First THT Application: Colorectal Cancer Tumors
Why is THT uniquely suited for colorectal cancer treatment?
- Alternatives diminish quality of life
- Significant market
- Effective for solid tumors
- Early detection is possible THT can be integrated with “watch and wait” approach
- Low metastatic index
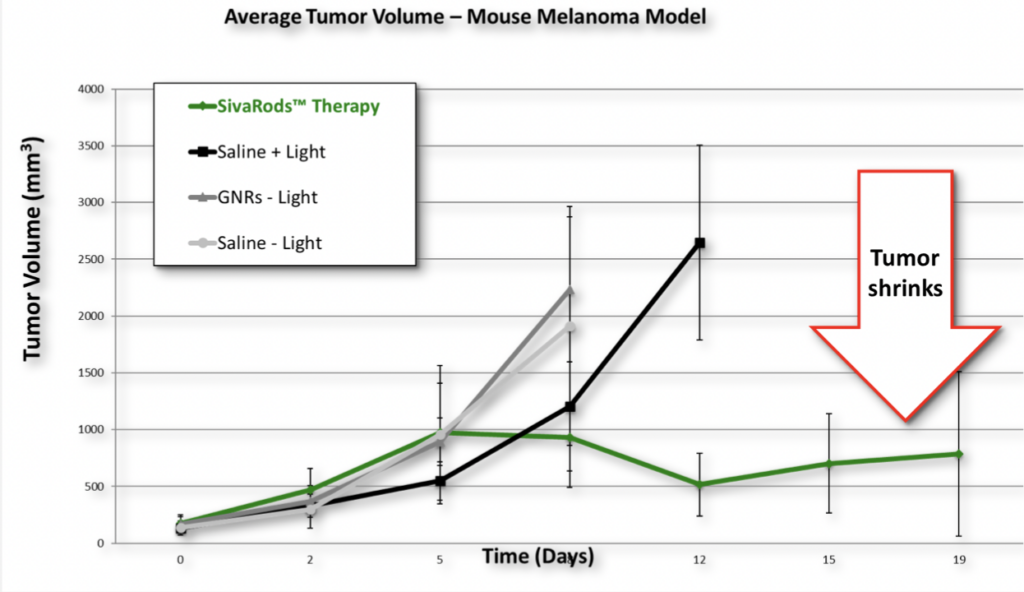
Pre-Clinical Success
- Photothermal therapy using gold nanorods and near-infrared light in a murine melanoma model increases survival and decreases tumor volume.


Catalysts Ahead
- Potential future clinical studies to provide multiple valuation catalysts
- Near-term catalyst in large animal studies (Preclinical)
- Success would initiate human pilot (Phase I)
Follow our Progress
- Join Sona Nanotech's Investor List
Leveraging GNR Gold Nanorod
Sona’s GNR technology in Siva Therapeutics’

Uniquely biocompatible

Unlocking of in-vivo medical applications potential

Current Cancer Treatments are risky, expensive and can do harm
- Chemotherapy and radiotherapy are non-selective in their destruction of cells;
- Advanced therapies are expensive;
- Surgery is risky.
Forward Looking Statement
This presentation contains forward-looking information under applicable securities law. All information that addresses activities or developments that we expect to occur in the future is forward-looking information. Forward-looking statements are based on the estimates and opinions of management on the date the statements are made.
Such forward-looking statements include, but are not limited to, statements regarding the future development of Targeted Hyperthermia Therapy and the anticipated timing and terms of Sona’s planned equity raises.
Forward-looking statements are necessarily based upon a number of assumptions or estimates that, while considered reasonable, are subject to known and unknown risks, uncertainties, and other factors which may cause the actual results and future events to differ materially from those expressed or implied by such forward-looking statements, including the risk that Sona and Siva may not be able to secure animal and human clinical studies, or develop the envisioned device or therapy, and the risk that equity financing may not be available on the anticipated terms or at all.
Actual results may differ materially from those set forth in this presentation due to risks and uncertainties affecting Sona and its products, including the demand for Sona’s tests which may be adversely affected by introduction or success of competing products, the ability for Sona to successfully develop longer-term applications for its technology and other risks detailed from time to time in Sona’s ongoing filings and in its most recent annual information form filed with the Canadian regulatory authorities on SEDAR at www.sedar.com.
Readers are cautioned not to place undue reliance on these forward-looking statements and are encouraged to read Sona’s continuous disclosure documents which are available on SEDAR. Such statements should not be regarded as a representation that any of the plans, expectations or intentions will be achieved. Sona takes no responsibility to update forward-looking statements in this presentation except as required by law.
How does THT work?
- Targets heat directly to the tumor;
- Uses IV injection of GNRs to heat the tumor from the inside;
- Achieves hyperthermia instead of ablation destroying cancerous cells selectively;
- Sona GNRs are inert and do not use toxic CTAB.
Targeted Hyperthermia Therapy™ (THT)
Medical device with two components:
- Gold nanorods for injection
- Infrared light source
Heating tumors does the following:
- Stimulates immune system
- Kills cancer cells
- Increases tumor perfusion
- Shrinks tumors

Healthy cells withstand heat stress, up to 52°C, typical with ablation therapies.
Not damaged by hyperthermia’s 44°C
THT destroys cancer cells while healthy cells can stay undamaged
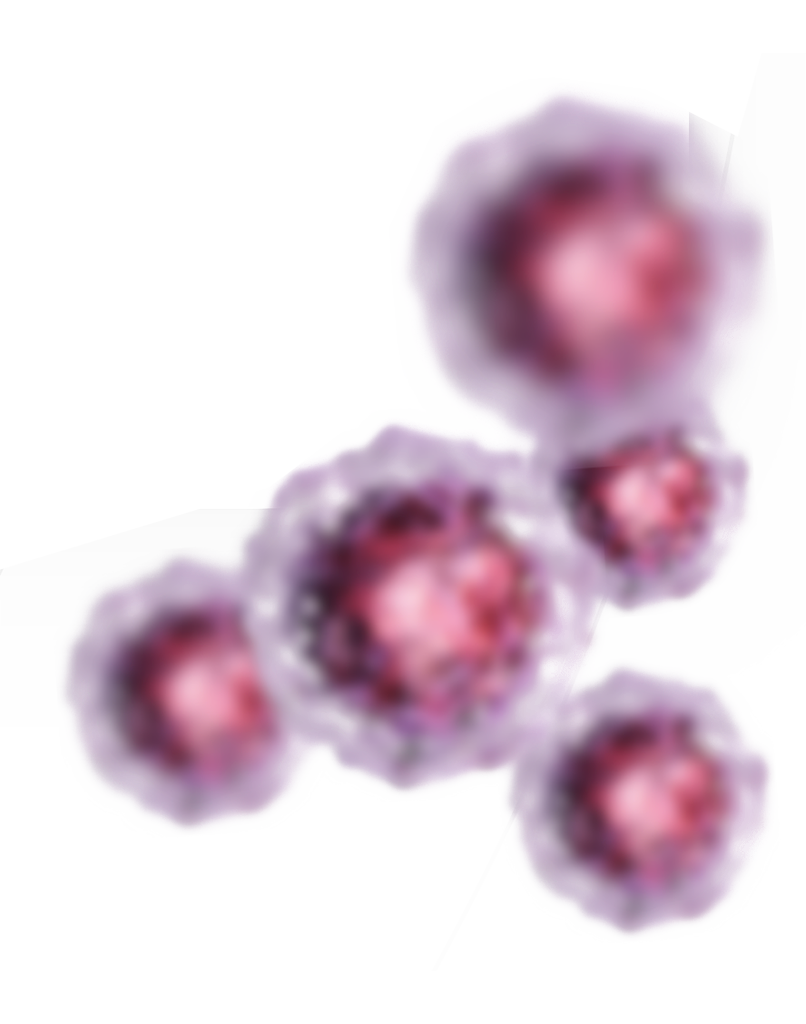
SivaRods™ heat to 44°C
Selectively kills cancer cells
Works from the inside of the tumor out
Heat shock protein (HSP) synthesis used

Cancer cells are more sensitive to heat
Siva’s Unique Differentiator:
Selective ‘Hyperthermia’ Minimizes Collateral Damage
- THT photothermal cancer therapy using GNRs will address current treatment issues.
- Siva's THT "inside-out" heating maximizes efficacy and minimizes collateral damage.

Hyperthermia
Blue Rare (115°F) i.e. 46°C
Hyperthermia doesn’t harm normal cells

Ablation
Medium (134°F) i.e. 57°C

Ablation therapies ‘cook’ proximate cells
- Siva has had major success in reducing tumors in small animal studies.
- Eliminated tumors in mice in 4 weeks
- Validated by Nanotechnology Characterization Laboratory study and report.
THT photothermal cancer therapy using GNRs will address current treatment issues
Key Issue
Using gold ‘in vivo’ is understood to be safe
Long-term effects of GNRs treated with toxic CTAB are unknown.
Sona GNR Advantage
Sona’s proprietary CTAB-free GNRs have shown no toxicity
Confirmed by third party and in-house testing

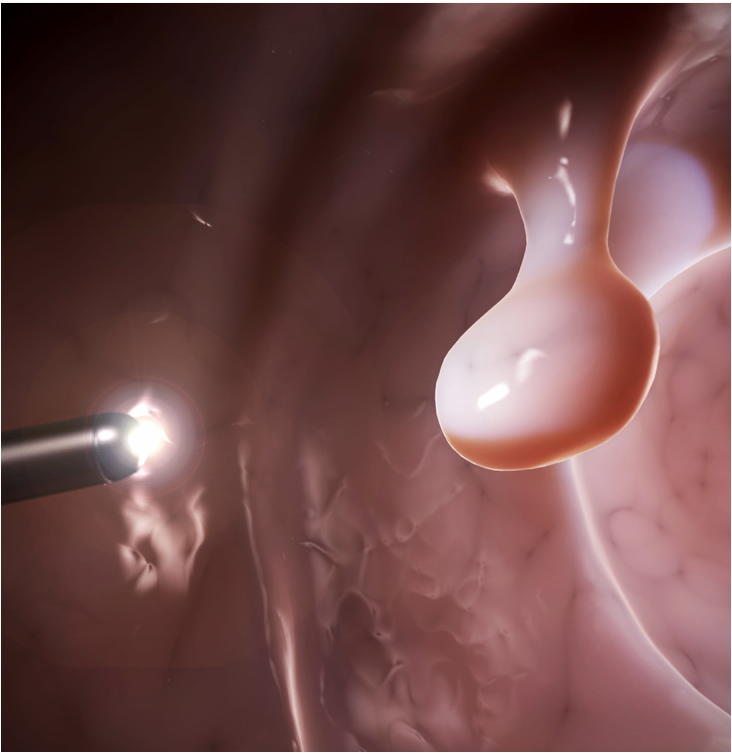



First THT Application: Colorectal Cancer Tumors
Why is THT uniquely suited for colorectal cancer treatment?
- Alternatives diminish quality of life
- Significant market
- Outpatient procedure within current workflow
- Effective for solid tumors
- Early detection is possible
- THT can be integrated with “watch and wait” approach
- Low metastatic index
Treatment Benefits:
- Minimally invasive
- Targeted treatment
- Enhances success of other cancer therapies
- Easy to use
- Affordable
Read More About Targeted Hyperthermia
- Preclinical Small Animal Study Success
- First THT Application: Colorectal Cancer Tumors
Road to Commercialization
Potential future clinical studies to provide multiple valuation catalysts.
Additionnal Publications